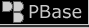





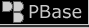 |
 |
 |
 |
 |
 |
Test strips are not my first choice in testing PG antifreeze concentration, but they are ok and can work. I used these test strips for illustration purposes because photographing through a sight refractometer is nearly impossible.
You can not effectively test PG antifreeze with an ethylene glycol tester. A sight refractometer is the best method but they are pricey. The good thing about a sight refractometer is they are also very, very accurate for testing the specific gravity of a battery so you kill two birds with one stone by owning one.
I often hear; "I just look for pink out the stern and shut her down."
While it may "show up", and you may "see pink", what is it's concentration before and after? What is the burst point? What is the freeze point? You may see pink but this does not necessarily mean you are protected against freeze damage for a real cold snap.
Water flow through a strainer, hoses, HX and water lift muffler is not simple complete displacement event. What you pump in gets mixed & diluted with whats already in there. -50F or -60F Propylene glycol should not get diluted or you will drastically raise your freeze and burst points. The -50 products are already heavily diluted and -50 is the burst point for metals not the freeze point.
The burst points for plastic are usually about 30F higher than for metals. Today many vessels ship with plastic sea strainers.
Add just a little water, through dilution, and your burst point raises very quickly to ranges we see up North in a regular winter.
I once watched a strainer while sucking in 5 gallons. The bottom of the strainer was still clear water at 3 gallons and by five gallons it was full purple. And this was at the intake strainer.
I know from testing that it takes a minimum of five gallons through our 44HP engine and RW circuit to get the same burst points out the stern as what it went in as. I also pre-drain the strainer which holds about 32 oz. The only way to know your burst point is to physically test what comes out or simply suck more in for added insurance.
Some engines will take less and some take considerably more. I depends a lot on the internal flow characteristics of your engine. We see plenty of freeze problems up North from too little AF used or improper winterizing techniques.
At $3.99 a gallon it can be a lot less expensive than freezing your strainer, raw water pump or heat exchanger.. Invest in a test kit then you'll know exactly how much your motor takes.
How do I test?
The absolute easiest method is to use a sight refractometer. These can also be used for testing the specific gravity of a flooded battery (actually my preferred tool for SG readings). The best way to use a refractometer is to take a baseline sample of the AF you are going to use. Look through the sight glass and read it. This is your baseline number. Now suck in one gallon of AF and capture a sample in a cup out the exhaust. Compare it to the baseline reading. Does it match?
What comes out the exhaust should match perfectly what went in. If you need more suck in another gallon and test again. Once you have hit your match, same in as out, you know exactly how much your engine takes to be adequately protected.
Every spring I deal with frozen and split heat exchangers, water heaters, valves etc. all from owners allowing the concentration to become far too diluted.
NOTE: Winterizing raw water cooled engines very often requires the removal of the thermostat for effective freeze protection. If unsure consult someone who knows your engine well.
© All Images property of Compass Marine Inc.